Background: Veno-occlusive disease (VOD) is a rare but potentially life-threatening complication following allogeneic stem cell transplantation (allo-SCT). Improved transplant techniques and increased awareness of modifiable risk factors have reduced VOD incidence. However, the interaction of historic risk factors in the current era, particularly with the increasing use of calicheamicin-based therapies and post-transplant cyclophosphamide as GVHD prophylaxis, remains unclear.
Methods: We conducted a retrospective, single center, chart-based study of consecutive adult patients (age ≥ 18 years) undergoing allo-SCT at MD Anderson Cancer Center between January 1 st, 2017 and December 31 st, 2021. VOD cases were defined using the classic VOD EBMT criteria or if patients received defibrotide treatment for VOD based on clinical judgment of the treating physician. Risk factors for VOD were evaluated in univariate analysis using Fine and Grey regression analysis. Multivariate analysis was performed using Classification and Regression Tree (CART) analysis to validate the findings of univariate analysis and evaluate independent effects accounting for potential interaction effects.
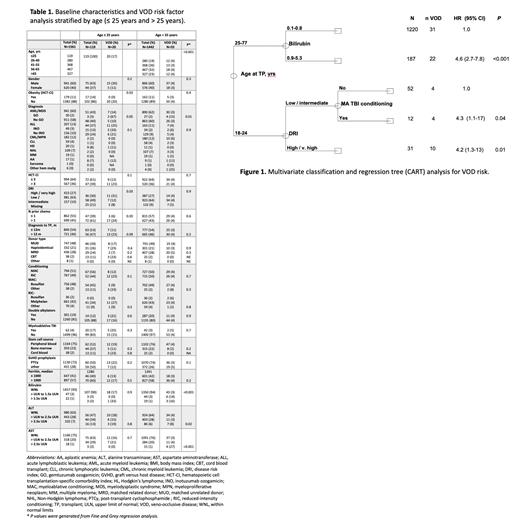
Results: A total of 1561 patients were included in our analysis. Median age was 56 years and 60% were male. Patients primarily had acute myeloid leukemia/myelodysplastic syndrome (AML/MDS; 60%), acute lymphoblastic leukemia (ALL; 13%), or myeloproliferative neoplasms (13%). Most patients had matched related (28%) or unrelated donors (48%), and 21% underwent haploidentical transplantation. Forty-nine (3%) patients received inotuzumab ozogamicin (InO) containing regimens prior to allo-SCT, and 30 (2%) were exposed to gemtuzumab ozogamicin (GO). Post-transplant cyclophosphamide (PTCy) was used as GVHD prophylaxis in 72% of patients. Incidence of VOD in the entire study population was 4.8%. There was a significant difference in the median age of patients diagnosed with VOD compared to those without VOD (46 years vs 56 years, respectively, p=0.001). Further examination of the incidence of VOD within different age groups identified a VOD rate of 16.8% (20/119) in those aged ≤ 25 years compared to 3.8% (55/1442) in those > 25 years (Table 1). Multivariate classification and regression tree (CART) analysis confirmed age as the primary independent determinant of the rate of VOD (Figure 1). Given these findings, risk factor analysis was stratified according to age.
In patients ≤25 years of age, disease risk index (DRI) (31% with high/very high DRI vs 12% low/intermediate DRI; p=0.03) and prior lines of chemotherapy (24% with >1 vs 6% with ≤1, p=0.03) were the strongest predictors of VOD. Within the younger cohort of patients with AML/MDS, 2 of 3 patients (67%) who received gemtuzumab ozogamicin (GO) developed VOD compared to 10% who did not receive GO (p=0.05). In patients aged >25 years, elevated baseline bilirubin, AST, ALT, and GO exposure were associated with increased VOD rates. VOD incidence based on baseline bilirubin levels (WNL, >ULN to 1.5x ULN, and >1.5x ULN) was 3%, 14%, and 16%, respectively (p<0.001). For baseline ALT levels (WNL, >ULN to 2.5x ULN, and >2.5x ULN), VOD rates were 4%, 3%, and 8%, respectively (p=0.02). Similarly, VOD rates with baseline AST levels (WNL, >ULN to 2.5x ULN, and >2.5x ULN) were 3%, 4%, and 27%, respectively (p≤0.001). In patients aged >25 years with AML/MDS, those receiving GO had a higher incidence of VOD compared to AML/MDS patients not exposed to GO (15% vs. 3%; p=0.01). There was no significant difference in VOD rates between those receiving PTCy and those receiving alternate GVHD prophylaxis in either age cohort.
Conclusion: In summary, our data highlight that young adults (age 18 - 25 years) represent a distinct adult population with increased rates of VOD compared to their older counterparts, which are exacerbated by disease and treatment related factors (DRI and number lines of therapy). In contrast, patients > 25 years of age had low rates of VOD even in the presence of historical predictors, with only hepatic risk factors identified as increasing baseline VOD risk. There was no observed increased incidence of VOD among those receiving PTCy as GVHD prophylaxis.
Disclosures
Bashir:Stemline: Research Funding; Acrotech: Research Funding; GSK: Research Funding; Pfizer: Research Funding. Qazilbash:NexImmune: Research Funding; Janssen: Research Funding; Angiocrine: Research Funding; Amgen: Research Funding; Bioline: Other: Advisory board. Nieto:Affimed: Research Funding; Secura Bio: Research Funding; Astra Zeneca: Research Funding. Marin:Affimed: Patents & Royalties; Takeda: Patents & Royalties. Rezvani:Affimed: Other: License agreement; Takeda: Patents & Royalties. Khouri:Pfizer: Research Funding. Srour:Orca Bio: Research Funding. Champlin:Arog: Consultancy; Orca Bio: Consultancy; Actinium Pharmaceuticals: Consultancy; Kadmon: Consultancy; Johnson & Johnson/Janssen: Consultancy; Omeros: Consultancy; Cell Source: Research Funding; Takeda Corporation: Patents & Royalties. Shpall:Axio: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: License agreement; Affimed: Other: License agreement; Syena: Other: License agreement; Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Navan: Membership on an entity's Board of Directors or advisory committees; NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees. Kebriaei:Jazz: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal